
December additions in SmartChem
New Additions
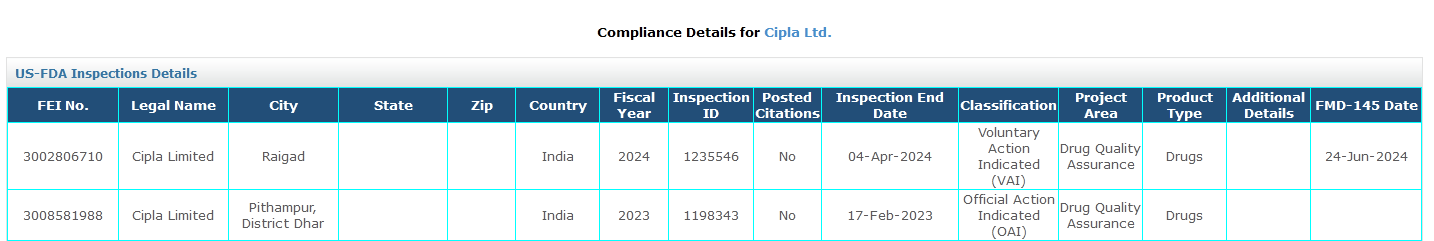
1. US FDA Inspections Details
You can find US FDA inspections on the company page with vital information such as the inspection end date and FMD_145 date, along with the classification remark. You will find this information under the GMP Compliance tab on the company page.
2. US FDA form 483
It is available in the non-compliance tab on the company page. We have added the link so that you can review the entire report at your convenience. The data for 2023-2024 is available on SmatChem.
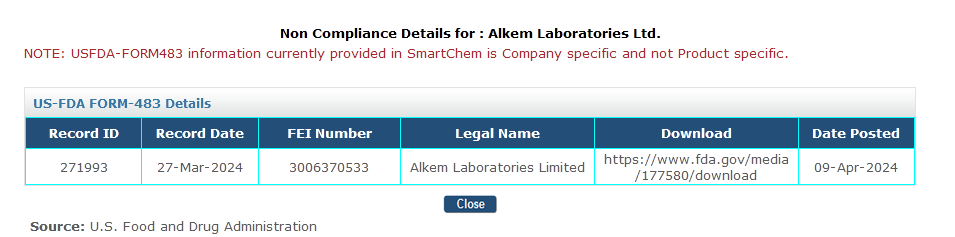
With these two additional features and the Warning Details, you will gain a comprehensive overview of FDA inspections. This will greatly benefit your business development efforts in the regulated market.
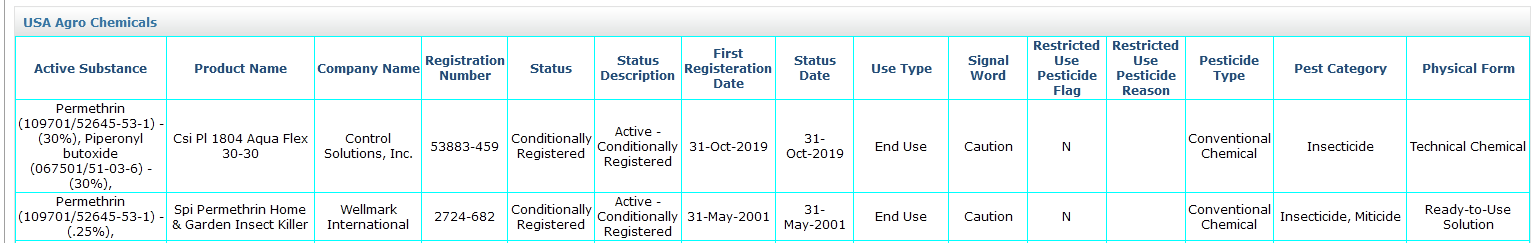
3. US Agrochemical Registration
You can now find US Agrochemical Registration data in SmartChem, which includes crucial information like agrochemical compositions, important dates, physical forms, trade names, etc.
4. Pie chart
On the supplier and formulators page, we have added a pie chart representing all the types of suppliers. This graphical representation will give you a better understanding of all the suppliers present at one glance.

5. Formulators Summary
Now, when you click on the formulators tab, you will find a summary at the top with the number of the types of formulators present.
We’re excited about these updates and can’t wait for you to explore them! If you have any questions or need assistance, we’re here for you every step of the way.
